The World Health Organization says we’re currently losing to the bugs. Because resistance to antibiotics is increasing; and because there are too few new antibiotics in the pipeline. In The Conversation, Allen Cheng reviews the situation.
Antibiotics, a wonderful discovery
We tend to think that antibiotics have been around forever. But they are being used for treatments less than one hundred years. They’ve been miraculous. Before their discovery, patients might die of trivial infections. In other cases, like tuberculosis, if people didn’t die at short notice, their bodies would be eaten away slowly over many years. But once we had antibiotics, the outcomes for these infections were much better.
Antibiotics were discovered accidentally by Alexander Fleming in 1928. But it wasn’t until 1941 that the first patient could be treated. On this site, we wrote an entire history of antibiotics, check here. At present, the world is full of antibiotics. So much so that they are gradually losing their effectiveness. Leading us to the question: what next?
The problem of overuse
We use antibiotics for a number of reasons, Allen Cheng says. They reduce the duration of illness and the chance of death from infection. If one is undergoing surgery or has a weakened immune system, the antibiotic will prevent an infection. But antibiotics aren’t always used appropriately. Studies consistently show a dose or two will adequately prevent infections after surgery, but antibiotics are often continued for several days unnecessarily. And sometimes we use the wrong type of antibiotic.
Surveys have found 22% of antimicrobial use in hospitals to be inappropriate. Doctors often make use of broad-spectrum antibiotics in order to make sure that the patient doesn’t catch another infection. And doctors suffer from inertia as well. If the patient is improving, they will tend to continue the existing treatment. Overprescription is a common phenomenon. This is particularly true for the use of antibiotics in animals. They are used, partly as a means of growth promotion, partly as a general shield to any infections passing by.
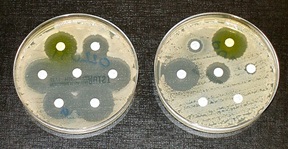
Resistance
But overuse will cause resistance. Through the phenomenon of natural selection, resistant strains of bacteria will eventually survive. What to do when this happens, and the treatment doesn’t have any effect anymore? The doctor’s natural reaction will be to revert to a stronger antibiotic. But stronger antibiotics often also have stronger side effects. Setting in motion a continuous chain of using more severe substances. In some countries, the pressure on doctors to continue prescribing more powerful medicines can be very hard. But this will also tend to reduce the number of effective medicines at the doctors’ disposal: as each antibiotic will eventually produce its own resistance.
On the other hand, doctors may also be reluctant to prescribe new antibiotics. This will have an effect on the development of new medicines, as such a reluctance will reduce the use of the new medicine. It will be regarded as a last resort and mainly remain on the shelf – which is killing for its earning power to the production company. The doctor’s attitude is very understandable: less use will prolong the effectiveness of the new antibiotic. But this very logic will also reduce the willingness of companies to develop new drugs.
All in all, antibiotic resistance is very gradually becoming a major problem. Antibiotic resistant bacteria are on the rise. Cheng reminds us of the fact that in 2019, almost 5 million deaths occurred with an infection involving antibiotic-resistant bacteria. And this will get worse. And it is estimated that by 2050, deaths from antimicrobial resistance could rise to 10 million each year, globally. This phenomenon might then cost 2-3.5% of global GDP.
So, what to do?
Cheng closes his article by recommendations that we reproduce in their entirety. ‘There is a lot we can do to prevent antibiotic resistance,’ he writes. ‘We can:
- raise awareness that many infections will get better by themselves, and don’t necessarily need antibiotics
- use the antibiotics we have more appropriately and for as short a time as possible, supported by co-ordinated clinical and public policy, and national oversight
- monitor for infections due to resistant bacterial to inform control policies
- reduce the inappropriate use of antibiotics in animals, such as growth promotion
- reduce cross-transmission of resistant organisms in hospitals and in the community
- prevent infections by other means, such as clean water, sanitation, hygiene and vaccines
- continue developing new antibiotics and alternatives to antibiotics and ensure the right incentives are in place to encourage a continuous pipeline of new drugs.’
All in all, de-risking the use of antibiotics is a matter of deliberate action; not always easy in the patient-doctor interaction. And of continuous oversight.
The situation isn’t hopeless
But in spite of all these cautions, seven out of seven global experts interviewed by The Conversation, judged that we will still have antibiotics in 50 years’ time. The measures they advocate include the ones mentioned above. And:
- use viruses that naturally kill bacteria, stimulating the host’s immune system to fight the bacteria
- combine existing antibiotics with molecules that can enhance antibiotic activity by, for example, increasing uptake or blocking resistance
- develop unnatural compounds that can circumvent the evolution of antibiotic resistance: by use of AI, machine learning, and other computational tools
- limit the ability of bacteria to cause disease or evade our immune systems, by the development of narrow-spectrum medicines
- more attention to the development of antimicrobial activity in developing countries
- all in all, more political attention to the problem, with an eye on the local context
- and the continued development of new drugs, like new antibiotics, viruses that kill bacteria, specific antibodies, drugs that counter antibiotic resistance; also assisted by advances in computer technology.
We are definitely entering into a new age of disease control. But the situation isn’t hopeless. Yes, the times of careless application of new drugs are over. We need to be much more aware of what we do. And develop new research approaches. But that said, we will still be able to control many diseases in the near future. Be it with more care, and more specificity in our actions.
Interesting? Then also read:
New antibiotics – their development stagnates. Why?
Antibiotics resistance, and how to overcome it
Chemistry vs. antibiotics, # 57. Phage therapy, a promising alternative to antibiotics?
