The serious search for new antibiotics starts only in 2015. Private and public initiatives collect funds for new research. Oxford University for instance, equips a new institute for research and action on resistance. The results seem to be positive. Between 2016 and 2020, 14 new tools are admitted to the market. Did we find the solution here?
Project ‘100 years of antibiotics’
Episode 50. Resistance, a second look
Episode 51. New antibiotics, how not to proceed. The story of lefamulin
Episode 52. Prospect of new tools??
Episode 53. If chemistry loses
Episode 54. If chemistry and biotech join forces
In the first 20 years of this century, the American FDA has admitted 650 new medicines to the market. That number included few antibiotics: less than 40 new tools against bacterial infections. Moreover, most substances in this class are mere variations on last century’s themes; they add little therapeutic value. Their main intended function is to minimize resistance to the old varieties. Really new products are rare, as are new concepts to attack the pathogenic organisms.
For the time being, the initiatives since 2015 do not deliver much. More of the same. Medicines with little added value, according to the WHO. And a few promises in very early development phases.
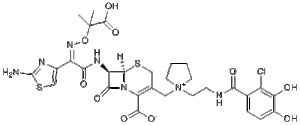
New promises
In the category of the cehalosporins, an important new substance is cefiderocol (2019), produced by the Japanese company Shionogi. This compound makes use of a new mechanism to deliver the medicine to the bacterium. The catechol side chain (at the far right of the formula) binds well to iron, and hikes into the bacterium, using the pathways available to the bacterium for importing iron into the cell. A new Trojan horse.
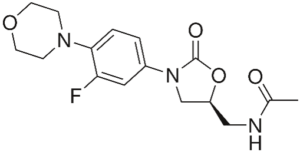
We already discussed the oxazolidones in episode 38, with linezolid as their most important representative: much ado about nothing. Tedizolid (2014) is a more active successor, but itself not a really new substance either.
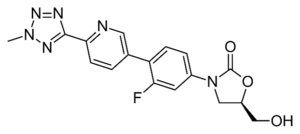
The large streptomycin family has produced a new subclass of antibiotics: the streptogramines; with representatives like quinupristin, dalfopristin and pristinamycin. They are products preferably used as agents of last resort, for instance if vancomycin fails to contain the infection. The origin of substances lies in their application as an additive to fodder. They are made by the bacterium Streptomyces virginiae.
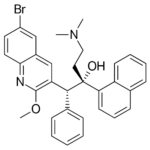
The quinolones, mentioned earlier, also produce a new sub class: the di-aryl-quinolones, with bedaquiline as their best known representative. All of them purely synthetic medicines. Bedaquiline is mainly used in the treatment of tbc, when the patient appears to be resistant to most common medicines. Its mode of action against the bacterium is new. It blocks ATP production in the cell. ATP is well-known to be one of the most important molecules for transporting energy within the cell. But there is a major concern. ATP production in our own cells resembles much that in the tb bacterium. Therefore, this medicine might lead to serious side effects.
And researchers find a new class of substances with bactericide action in microorganisms that produce macrolides (like the erythromycines). The first example is fidaxomicin. This is mainly used to cure diarrhoea caused by the infamous Clostridium difficile. The main advantage of this substance is that it is fairly specific, and doesn’t influence much the other flora of the intestinal tract. The fidaxomicin cure is one of the most expensive cures available.
All in all, it seems that we can only look upon the pleuromutilins as fundamentally new antibiotics. In episode 51 we discussed extensively the sad story of their development.
New tools in the pipeline
But the race for new tools is now seriously coming on stream. There is a general fear of resistance, and it makes itself felt; stimulation programs by several authorities also fan the interest. But it is almost impossible to predict which molecules will be a market success – there are dozens, if not hundreds of them right now. Remember, rational design of a medicine has remained a utopic concept for over a century; and that also holds true for predictions on the basis of what is in the pipeline now.
Still, we mention a few likely candidates. Some researchers produce synthetic derivatives of the natural product clindamycin; these circumvent a number of resistance mechanisms. But the problem is that we don’t quite know how they go about this. A promising product might be iboxamycin (see C&E News, 10-28-2021). Other researchers use smart additions to an existing antibiotic in order to circumvent the bacterial resistance mechanisms, and smuggle in the substance anyway. Trojan horses again. Makes good reading, but it is hard to judge the value of such developments. Like the combination of a contrast fluid with an existing antibiotic. The contrast fluid contains, among others, a radioactive Gallium isotope; therefore, we can easily follow its course in the body together with the antibiotic. Moreover, the bacterium regards the gallium metal as being iron, and therefore imports it through its proper pathway.
Imaginative are new ways to puncture holes into the cell wall. For instance, researchers constructed synthetic polymers of laevo and dextro amino acids that together form a sharp needle and puncture the hole. But then, we will have to be sure that it’s the bacterial cell wall they puncture.
New concepts
As we remarked before, existing antibiotics target a limited number of goals in the bacterial cell: the cell wall, the DNA and the RNA machinery, the production of a number of proteins and the disruption of the beta lactamases. But the cell has hundreds of weak points, of which dozens might constitute a target for antibiotics. Resistance mechanisms have been investigated in detail as well. All parts of them can serve as targets for agents that disrupt that mechanism. For instance, bacteria under stress may produce the toxic gas hydrogen sulphide. That promotes clinging together of bacteria to form a biofilm. That is a survival mechanism. Antibiotics can hardly penetrate such a film. The new tools aim to block the enzymes that regulate hydrogen sulphide formation. The enzyme in question is available in crystal form and can be studied in each part of its activity. Likewise, we can now see the locations where another product may block the enzyme’s mode of action. Using such tools, researchers can test the entire inventory of existing medicines on their computers; and discover which options they have for the prevention of hydrogen sulphide formation.
And then, a lot is going on in the methods we use to test potential new tools. We would preferably test them safely and quickly on relevant living organisms (i.e. animal testing). Right now, the distance is too far from the testing target (often in a test tube) to the eventual target (the patient). Do we have to refer to animal testing then? We would like to end that as well. But we manage better now to test on living cells; or on organoids (a small number of cells that together act as an organ); or on live models that raise less ethical problems, like the 1 mm long roundworm C.elegans, that has many properties in common with larger animals (and with humans), as far as their reactions on antibiotics are concerned.
Back to nature
Nature still is the ultimate source of new molecules with possible medicinal properties. We find such substances in every search; be it deep in the oceans, on remote locations, in plants and insects and particularly in microorganisms. It has been estimated that we have just researched 10% of all living creatures on earth. Much less if microorganisms are concerned. For 99% of them, we don’t know how to cultivate them. Meaning: we can isolate the organism from nature and study it, but we don’t know how to grow the organism, or how to let itself reproduce.
But then, we might also take a look at ourselves. We learn to know more and more about the way in which our immune system reacts to intruders. That might inspire new designs of antibiotics. And we don’t have to restrict ourselves to the immune system. Many components of our bodies react to intruders, and the mechanisms employed there may also put us on new tracks. Or consider the house fly; after a bacterial infection it knows what food to avoid.
What about commercial prospects?
In the sector, many people reflect on the sad story of the pleuromutilins. Sadly, research on medicines has become too expensive. In 2020, the sector follows up on this insight by creating a fund of about 1 billion dollars; intended to carry medicines through the expensive phases of research. Big names are involved: Pfizer, Novartis and Johnson & Johnson. But is this amount sufficient to serve the goal? In view of looming bankruptcy because successful medicines stay on the shelves? The developers of pleuromutilin aren’t the only ones that suffered. At least three small companies ended in bankruptcy before them: Ardigem, Achaogem, Melinta. This implies that there should also be some safeguarding of sales and profits. How can we go about this? The UK tries to develop a new model of reimbursements that uncouples profit and sales. In the form of contracts for several years between production and insurance companies. With a reimbursement dependent on better health care for the insured, as judged by experts.
The US, Sweden and Germany try to develop such a scheme as well. For their success, these schemes will require international cooperation and consultation. The Covid pandemic has now moved such discussions about antibiotics to the background. But precisely in this pandemic, authorities have developed new methods for funding or reimbursing corona vaccines on beforehand. That might also lead to reimbursement mechanisms for antibiotics, acceptable to all parties involved. Anyway, these matters will never become simple again, as they were before.
Sources:
Wikipedia: all names and products mentioned
List of FDA approved medicines 1999-2021
Antibiotics and bacterial resistance in the 21st century, R.J. Fair and Y. Tor, Perspectives in Medicinal Chemistry 6, 25-64(2014)
Striving to bolster the antibiotic pipeline before it becomes the next crisis, Katrina Megget, Chemistry World 26-5 2021
